Sažetak
The “standard model” of electroporation represents transport through pores formed when the transmembrane voltage exceeds a critical value. Although the model has evolved from the transient, stochastic pore scheme proposed over 30 years ago, it still does not predict key features of electropermeabilized cells, including: pore lifetimes; dynamics of membrane potential, electrical conductivity, and permeability to small molecules; multiple‐pulse protocol outcomes; effects of cell size and medium conductivity.
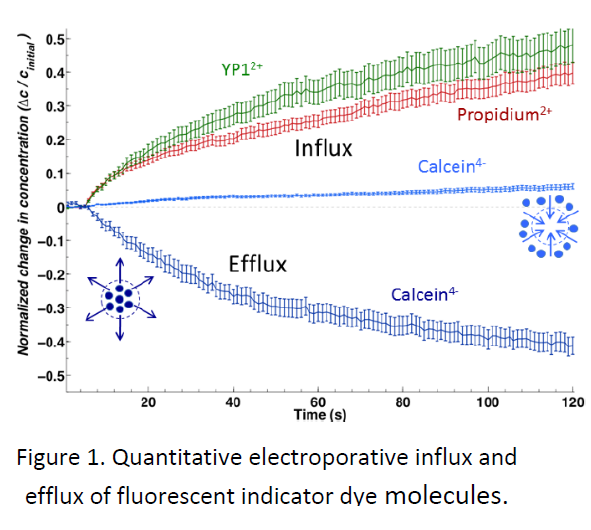
Impermeant, fluorescent small molecules serve as convenient indicators of electroporation. Among the most frequently used are propidium, calcein, and YO‐ PRO‐1. Similar in size, these three molecules show strikingly different patterns of entry into cells after exposure to permeabilizing electric pulses. These differences are not predicted by the standard model, and until now they have escaped notice in the electroporation literature.
We describe experiments that highlight the importance of the charge of these molecules (propidium and YO‐PRO‐1 are positive; calcein is negative), and which are consistent with electrophoretic transport of charged, normally impermeant small molecules after membrane permeabilization. Conventional wisdom holds that the transmembrane potential after electroporation is so small that it can be ignored—the dogma of the conductive membrane. We point to recent evidence for rapid recovery of the resting membrane potential, and we show that transmembrane potentials as low as 10 mV must be taken into account in studies of the post‐pulse transport of charged species across the membrane.
Recovery of membrane resting potential is only one of many cellular processes involved in the maintenance of homeostasis. In addition to generating a transmembrane potential, a cell perturbed by membrane permeabilization must also activate volume and osmotic balance regulation, membrane repair, Ca2+ and Na+ efflux, K+ uptake, and increased ATP production (needed not only for the additional demands of stress response but also to replace the ATP that leaks out through the compromised membrane). We know little about what we call the electropermeome—the interlinked networks of permeabilizing structures and processes and the stress response mechanisms activated by the breakdown of membrane barrier functions. But to be predictive beyond the initiation of permeabilization, electroporation models must represent this complexity, integrating the immediate physics of electropore formation with the subsequent physical, chemical, and biological responses of cells to the stress of membrane disruption.
P. Thomas Vernier is Research Professor at the Frank Reidy Research Center for Bioelectrics at Old Dominion University in Norfolk, Virginia, USA. His research and industrial experience includes ultraviolet microscopy analysis of S‐ adenosylmethionine metabolism in a psychrophilic strain of the yeast Rhodotorula glutinis, molecular biology of the temperature‐sensitive host restriction of bacterial viruses in Pseudomonas aeruginosa, low‐level environmental gas monitoring, wide‐band instrumentation data recording, physical and electrical characterization and modeling of semiconductor and microelectromechanical devices, and the integration of cellular and biomolecular
sensors, carbon nanotubes, and quantum dots with commercial integrated electronic circuit fabrication processes. Vernier currently studies the effects of electric fields on biological systems, with applications in cancer therapeutics, combining experimental observations with molecular dynamics simulations. His focus is on understanding the biophysical mechanisms that govern electric field‐driven, nondestructive perturbations of biological membranes.
Vernier received his Ph.D. in Electrical Engineering from the University of Southern California (USC) in 2004 and was for 10 years a member of the research faculty in the Ming Hsieh Department of Electrical Engineering at USC and Engineering Manager of the semiconductor fabrication service MOSIS. His professional associations include the American Chemical Society, American Society for Microbiology, Bioelectrochemical Society, Bioelectromagnetics Society, Biophysical Society, European BioElectromagnetics Association, International Society for Electroporation‐Based Technologies and Treatments, and Institute of Electrical and Electronics Engineers. He serves on the faculty of the annual international course on electroporation‐based technologies and treatments at the University of Ljubljana and maintains active collaborations with investigators in Barcelona, Bordeaux, Buenos Aires, Cambridge (Massachusetts), Cluj‐Napoca, Copenhagen, Golm, Halifax, Karlsruhe, Limoges, Ljubljana, Los Angeles, Merced (California), Naples, New Haven (Connecticut), Paris, Reno (Nevada), Rome, Santa Barbara (California), Toulouse, and Zagreb.
*ZAJEDNIČKI SEMINAR Hrvatskog biofizičkog društva i Zavoda za istraživanje mora i okoliša
![]()